Life
Sciences
We provide timely and accurate drug development and commercialization services from our U.S. owned and operated CDMO/CRO network.
improving our health
We believe the therapies our customers develop are critical to improving lives and we are proud to be a part.
Future role of Pace® Life Sciences in supporting biopharmaceutical development and manufacturing coast-to-coast – Dean Bornilla, Vice President.
With a growing network of locations across major US pharmaceutical hubs, Pace® aims to enhance customer experience through consistent quality and timely delivery.
Pace® Life Sciences Awarded Bronze Medal from EcoVadis for Commitment to Sustainability
Pace® Life Sciences, is pleased to announce that it has received a Bronze Medal award from EcoVadis for its current sustainability initiatives. This recognition places Pace® in the top 35% of over 130,000 companies evaluated for their commitment to sustainability.
Addressing the Complexity of Modern Therapeutics – Dr. Frank Tagliaferri, Pace® Life Sciences
The modern therapeutic landscape is defined by an ever-growing portfolio of complex drug products – from biologics and gene therapies to mRNA-based vaccines and monoclonal antibodies.
Pace® Life Sciences to Expand Large Molecule Development, Manufacturing, and Analytical Testing Capacity in 2025
Company expands sterile fill-finish services, capacity for biologics development and manufacturing, and more.
Pace® Life Sciences Expands Capacity and Capability with Acquisition of Catalent’s
Analytical Services Laboratory in Research Triangle Park
Company broadens service offerings and strengthens network by purchasing Catalent’s Center-of-Excellence for Small Molecule Analytical Services facility in North Carolina
Explore our range of services
Wherever you are in your drug development journey, we can help.
THE RIGHT PARTNER FOR YOUR PROJECT
We have a nationwide network of state-of-the-art facilities, each with long-established histories of successful product development and commercialization and excellent audit outcomes from regulatory agency and client reviews.
FDA
REGISTERED
DEA
DEA Schedules I – V
GMP
COMPLIANT
ISO
17025 ACCREDITED
OUR PROMISE TO YOU
We honor our commitments so you can honor yours™. Our investment in state-of-the-art facilities and highly trained experts emphasizes our commitment to delivering positive customer experiences across all phases of pharmaceutical and biopharmaceutical development.
- RELIABLE DELIVERY
- COLLABORATIVE RELATIONSHIPS
- EXCEPTIONAL SERVICE
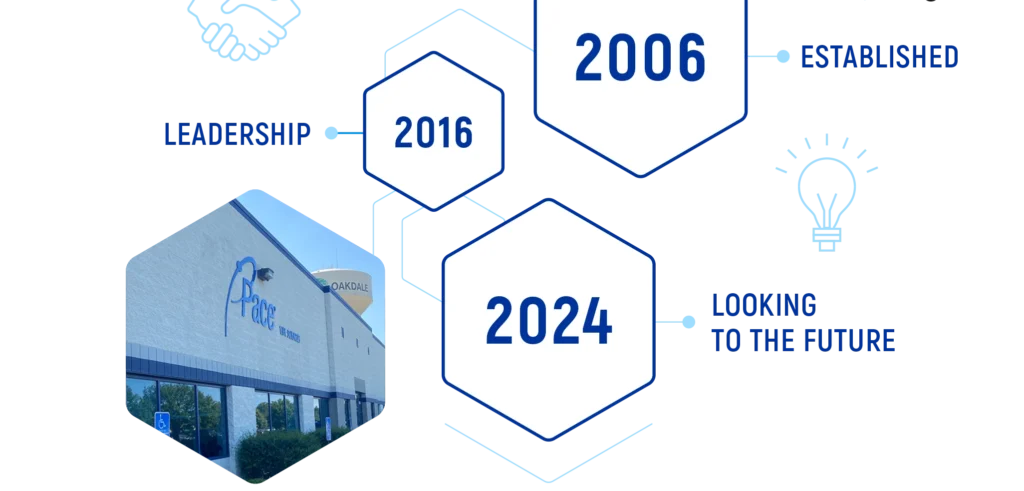
IT ALL STARTED IN 2006
Since 2006, Pace® Life Sciences has continued to prioritize strategic investments and domestic acquisitions to meet the changing needs of our customers. As the market changes, we are committed to making sure we are positioned as the best U.S. owned and operated end-to-end solution for your program.
Conferences

CPhI North America
Uniting the Americas’ pharma pioneers, under one roof from development to delivery.
May 20-22, 2025 | Philadelphia, PA
feature webinar

Overview of FDA’s Expedited Programs for Serious Condition
Recognize the importance of expedited review programs, such as FTD, BTD, and RMAT Designation, and their impact on reducing drug development time.
Now available on demand








